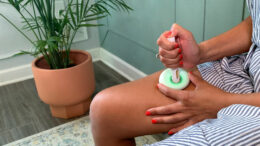
Noble can help patients reduce errors when self-administering biosimilar therapies
In today’s evolving healthcare environment, it’s not surprising that biosimilars have become more widely prescribed by physicians as an affordable alternative to biologics, which have been estimated to account for… Read More »
7 recommendations to increase training consistency for patients self-injecting biologics
Biologic drugs are finding growing success in addressing chronic diseases — from diabetes and rheumatoid arthritis to multiple sclerosis and Crohn’s. A significant rise in self-injection therapies is contributing to… Read More »
Supporting Patients During Treatment
We speak with Adam Shain, Director Business Development, Digital Healthcare, and Josh Hopkins, Program Manager, AdhereIT about the AdhereIT 360 Base and AdhereIT Clip – a runner up for The… Read More »
How Digital Medical Devices Are Meeting the Challenges Of Clinical Trials
The Internet of Things (IoT) – defined simply as a system of internet-connected devices that collect and transfer data over a wireless network – has transformed healthcare, from electronic health… Read More »
Noble Further Expands Capabilities of Its New Human Factors + Program
HF+ gaining momentum with additional offerings, successful support for new drug launches and expert hiring ORLANDO, Fla. (June 8, 2021) – In direct response to the rapid growth of the… Read More »