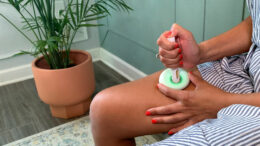
Supporting Patients During Treatment
We speak with Adam Shain, Director Business Development, Digital Healthcare, and Josh Hopkins, Program Manager, AdhereIT about the AdhereIT 360 Base and AdhereIT Clip – a runner up for The… Read More »
Noble/Aptar Pharma Digital Health Webinar
Joint Webinar from Noble & Aptar Pharma Discusses How to Improve Patient Adherence and Compliance With Innovative Digital Healthcare Solutions Hear from Adam Shain, Business Development Director, Digital Healthcare, Aptar… Read More »
Noble and Aptar Pharma Launch AdhereIT®, a Connected Medical Device Solution for Disease Management, Adherence and Onboarding Patients
Crystal Lake, Illinois and Orlando, Florida (October 5, 2020) — Noble and Aptar Pharma are pleased to announce the launch of AdhereIT® — a connected, intuitive and user-friendly onboarding solution… Read More »
AdhereIT 360 Base Demonstration Video
AdhereIT 360 Base Demonstration Video:
Enhancing Adherence to Self-Injection Regimens by Connected Devices
Many patients do not stick to their autoinjector regimens, which can lead to poor health outcomes. As Craig Baker, Executive Vice-President at Noble, explains, there are many reasons why this… Read More »
Connecting the Dots to Connected Health
As the mHealth market grows, the industry prepares to optimize digital opportunities. Measuring easily quantifiable data is one of the keys to better health. Therefore, the future belongs to digestible,… Read More »